Boiling Point Of 2 2-dimethylpropane
 | |||
 | |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name 2,2-Dimethylpropane[2] | |||
| Other names Neopentane | |||
| Identifiers | |||
| CAS Number |
| ||
| 3D model (JSmol) |
| ||
| Beilstein Reference | 1730722 | ||
| ChEBI |
| ||
| ChemSpider |
| ||
| ECHA InfoCard | 100.006.677 | ||
| EC Number |
| ||
| Gmelin Reference | 1850 | ||
| MeSH | neopentane | ||
| PubChem CID |
| ||
| UNII |
| ||
| United nations number | 2044 | ||
| CompTox Dashboard (EPA) |
| ||
| InChI
| |||
| SMILES
| |||
| Properties | |||
| Chemical formula | C five H 12 | ||
| Molar mass | 72.151 g·mol−i | ||
| Appearance | Colorless gas | ||
| Odor | Odorless | ||
| Density | iii.255 kg/mthree (gas, 9.v °C) 601.172 kg/one thousand3 (liquid, nine.5 °C) | ||
| Melting betoken | −16.5 °C (ii.3 °F; 256.6 Thousand) | ||
| Humid betoken | 9.five °C (49.i °F; 282.6 M) | ||
| Vapor pressure | 146 kPa (at 20 °C)[three] | ||
| Henry's constabulary | 4.vii nmol Pa−1 kg−1 | ||
| Thermochemistry | |||
| Heat chapters (C) | 121.07–120.57 J K−1 mol−one | ||
| Std molar | 217 J K−1 mol−1 | ||
| Std enthalpy of | −168.v–−167.three kJ mol−i | ||
| Std enthalpy of | −3.51506–−3.51314 MJ mol−1 | ||
| Hazards | |||
| GHS labelling: | |||
| Pictograms |   | ||
| Signal word | Danger | ||
| Take a chance statements | H220, H411 | ||
| Precautionary statements | P210, P273, P377, P381, P391, P403, P501 | ||
| NFPA 704 (burn down diamond) | i four 0 | ||
| Related compounds | |||
| Related alkanes |
| ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). Infobox references | |||
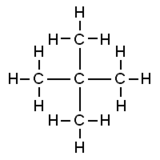
Neopentane, also called 2,2-dimethylpropane, is a double-branched-chain methane series with five carbon atoms. Neopentane is a flammable gas at room temperature and pressure which can condense into a highly volatile liquid on a cold day, in an ice bathroom, or when compressed to a higher pressure.
Neopentane is the simplest alkane with a fourth carbon, and has achiral tetrahedral symmetry. Information technology is one of the three structural isomers with the molecular formula CvH12 (pentanes), the other two being n-pentane and isopentane. Out of these three, information technology is the only one to be a gas at standard conditions; the others are liquids.
Nomenclature [edit]
The traditional name neopentane was even so retained in the 1993 IUPAC recommendations,[4] [5] only is no longer recommended according to the 2013 recommendations.[2] The preferred IUPAC proper noun is the systematic proper name 2,ii-dimethylpropane, merely the substituent numbers are superfluous because it is the but possible "dimethylpropane".

A neopentyl group fastened to a generic group R.
A neopentyl substituent, ofttimes symbolized by "Np", has the structure Me3C–CH2– for instance neopentyl alcohol (MethreeCCH2OH or NpOH). Every bit Np too symbolises the chemical element neptunium (atomic number 93) ane should use this abbreviation with care.
The obsolete name tetramethylmethane is also used, particularly in older sources.[half dozen] [7]
Physical properties [edit]
Boiling and melting points [edit]
The boiling bespeak of neopentane is just 9.5 °C, significantly lower than those of isopentane (27.7 °C) and normal pentane (36.0 °C). Therefore, neopentane is a gas at room temperature and atmospheric pressure, while the other 2 isomers are (barely) liquids.
The melting betoken of neopentane (−16.six °C), on the other hand, is 140 degrees higher than that of isopentane (−159.9 °C) and 110 degrees higher than that of n-pentane (−129.eight °C). This bibelot has been attributed to the meliorate solid-land packing causeless to be possible with the tetrahedral neopentane molecule; but this explanation has been challenged on account of it having a lower density than the other two isomers. Moreover, its enthalpy of fusion is lower than the enthalpies of fusion of both due north-pentane and isopentane, thus indicating that its high melting point is due to an entropy upshot resulting from higher molecular symmetry. Indeed, the entropy of fusion of neopentane is almost four times lower than that of n-pentane and isopentane.[8]
iH NMR spectrum [edit]
Because of neopentane'south full tetrahedral symmetry, all protons are chemically equivalent, leading to a single NMR chemic shift δ = 0.902 when dissolved in carbon tetrachloride.[ix] In this respect, neopentane is similar to its silane analog, tetramethylsilane, whose single chemical shift is zero by convention.
The symmetry of the neopentane molecule tin can be cleaved if some hydrogen atoms are replaced by deuterium atoms. In particular, if each methyl group has a unlike number of substituted atoms (0, 1, 2, and 3), ane obtains a chiral molecule. The chirality in this case arises solely by the mass distribution of its nuclei, while the electron distribution is even so essentially achiral.[x]
References [edit]
- ^ Aston, J.Thousand.; Messerly, G.H., Heat Capacities and Entropies of Organic Compounds II. Thermal and Vapor Pressure Data for Tetramethylmethane from 13.22K to the Boiling Signal. The Entropy from its Raman Spectrum, J. Am. Chem. Soc., 1936, 58, 2354.
- ^ a b "Front Thing". Nomenclature of Organic Chemical science : IUPAC Recommendations and Preferred Names 2013 (Blueish Book). Cambridge: The Imperial Society of Chemical science. 2014. p. 652. doi:x.1039/9781849733069-FP001. ISBN978-0-85404-182-four.
- ^ "Neopentane | C5H12 - PubChem".
- ^ Tabular array 19(a) Acyclic and monocyclic hydrocarbons. Parent hydrocarbons
- ^ Panico, R. & Powell, West. H., eds. (1994). A Guide to IUPAC Nomenclature of Organic Compounds 1993. Oxford: Blackwell Science. ISBN978-0-632-03488-eight.
- ^ Whitmore, Frank C.; Fleming, Geo. H. (1934-09-01). "Training of Tetramethylmethane (Neopentane) and Determination of its Concrete Constants1". Journal of the American Chemical Society. 55 (ix): 3803–3806. doi:10.1021/ja01336a058. ISSN 0002-7863.
- ^ LaCoste, Lucien J. B. (1934-10-fifteen). "The Rotational Moving ridge Equation of Tetramethylmethane for Zero Potential and a Generalization". Concrete Review. 46 (8): 718–724. Bibcode:1934PhRv...46..718L. doi:10.1103/PhysRev.46.718.
- ^ Wei, James (1999). "Molecular Symmetry, Rotational Entropy, and Elevated Melting Points". Ind. Eng. Chem. Res. 38 (12): 5019–5027. doi:ten.1021/ie990588m.
- ^ Spectral Database for Organic Compounds, Proton NMR spectrum of neopentane, accessed 4 Jun 2018.
- ^ Haesler, Jacques; Schindelholz, Ivan; Riguet, Emmanuel; Bochet, Christian Yard.; Hug, Werner (2007). "Accented configuration of chirally deuterated neopentane" (PDF). Nature. 446 (7135): 526–529. Bibcode:2007Natur.446..526H. doi:10.1038/nature05653. PMID 17392783. S2CID 4423560.
External links [edit]
- Linstrom, Peter J.; Mallard, William K. (eds.); NIST Chemical science WebBook, NIST Standard Reference Database Number 69, National Institute of Standards and Technology, Gaithersburg (MD)
- IUPAC Nomenclature of Organic Chemistry (online version of the "Blue Book")
Boiling Point Of 2 2-dimethylpropane,
Source: https://en.wikipedia.org/wiki/Neopentane
Posted by: tracyappot1978.blogspot.com




0 Response to "Boiling Point Of 2 2-dimethylpropane"
Post a Comment